EU MDR Now Regulates Magnificence Units As Medical Units
By Hilde Viroux, PA Consulting

The sweetness gadgets enterprise is booming. The worldwide worth of the beauty business amounted to over $530 billion in 2022, with an anticipated development price of roughly 4% over the following 5 years, whereas the sweetness gadgets market measurement stood at $66 billion and a growth rate over 20% through 2030.
Magnificence gadgets are merchandise which might be used on the face and physique for beauty causes, resembling, for instance, hair elimination gadgets, liposuction gadgets, and coloured contact lenses. The entry to the European markets has been straightforward for these gadgets because the Cosmetics Regulation 1223/2009 doesn’t apply to gadgets, since this regulation solely applies to pastes, lotions, and lotion kinds of merchandise.
The enterprise mannequin for magnificence gadgets is commonly based mostly on the “white label” mannequin, wherein distributors purchase the product from a producer and put their very own title and branding on it.
What Are The EU MDR Necessities?
The EU Regulation 745/2017 on medical gadgets (EU MDR) lists in Annex XVI a number of magnificence gadgets that the EU Fee desires to manage as medical gadgets. A key aspect for a product to qualify as a medical gadget within the EU is that it will need to have a medical profit. This medical profit should outweigh the dangers related to using the medical gadget. Magnificence gadgets don’t have a medical profit, as decreasing wrinkles or having smoother pores and skin just isn’t thought-about a medical profit. The EU Fee has now outlined ‘Frequent Specs” within the implementing regulation 2022/2346, which supplies necessities for the danger administration and labeling for every of the next specified teams of magnificence gadgets:
- Contact lenses, e.g., the so-called enjoyable lenses, nonprescription coloured contact lenses
- Beauty implants (other than breast implants, that are already thought-about medical gadgets)
- Beauty fillers, e.g., merchandise injected to plump up lips, cut back wrinkles
- Liposuction, lipolysis, and lipoplasty gadgets
- Mild emitting gear, resembling lasers or intense pulse gentle (IPL) gadgets for hair or tattoo elimination, pores and skin resurfacing, or different pores and skin therapies
- Mind stimulation gadgets and gadgets that apply electrical currents or electromagnetic fields that penetrate the skull to change neuronal exercise, e.g., to stimulate or affect moods
The gadgets get a threat classification per Annex VIII of EU MDR. Nevertheless, Regulation 2022/2347 defines the danger classification for among the gadgets the place the classification guidelines from the EU MDR will not be clear (marked beneath with *).
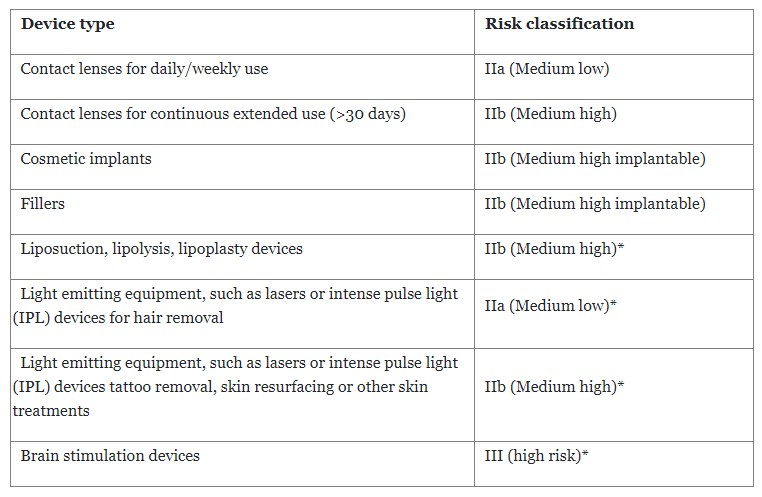
With the inclusion of those gadgets beneath EU MDR, producers not solely want to use the weather of threat administration and labeling as specified within the Frequent Specification but in addition must adjust to all parts of EU MDR — in different phrases, develop into de facto a medical gadgets producers.
Nevertheless, producers have a while to develop into compliant, based mostly on the danger classification of the gadget and relying on whether or not they need to conduct a medical investigation. The latter would be the case for gadgets which might be excessive threat (mind stimulation) or implantable (fillers, beauty implants).
What Are The Key Timelines?
The regulation gives a definition of the important thing timelines for compliance, relying on the kind of product:
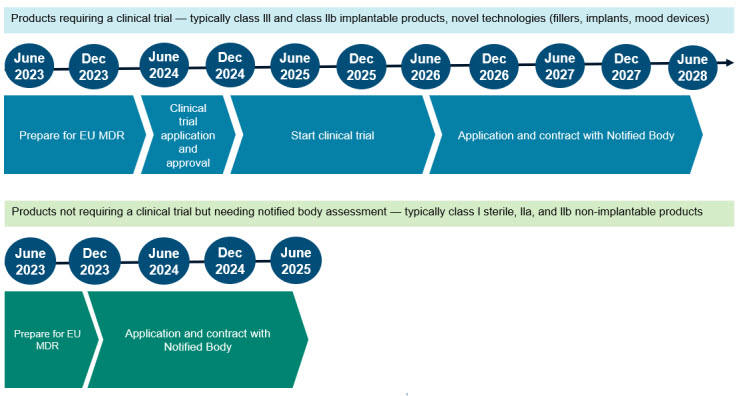
Units that want a medical trial as a part of the approval process, have till June 2028 for compliance, with the caveat that (1) the medical trial utility should be authorized earlier than December 2024, (2) the medical investigation is began earlier than June 2026, and (3) a contract with a notified physique is in place by June 2028. If any of the due dates usually are not met, the gadget could now not be offered within the EU.
For gadgets that don’t want a medical trial however do want the involvement of a notified physique for CE marking, the timelines are considerably shorter. The producer has between September 2023 and June 2025 to provoke the conformity evaluation with a notified physique.
What Are The Challenges For Producers?
December 2024 could appear to be loads of time to acquire approval for a medical trial utility. Nevertheless, earlier than the applying may be submitted, the design documentation and threat administration file should be accomplished, and an investigator brochure and medical trial utility should be ready and submitted. This may require new experience, both in-house or exterior. Two years appears a short while for finishing this, contemplating that the competent authority in-country additionally wants time to evaluation and approve the applying. A reliable authority that has no expertise with magnificence gadgets may have extra time than standard for evaluation and approval.
However that is solely part of the compliance train. Producers of those gadgets should totally adjust to all necessities of EU MDR. This implies a high quality administration system (QMS) that’s in keeping with ISO 13485, a design historical past file for the product, totally compliant technical documentation, and proactive publish market surveillance. This isn’t a straightforward endeavor, as evidenced by the massive variety of producers of normal medical gadgets that aren’t compliant with EU MDR (per Team-NB Sector Survey report, May 2022).
Turning into compliant with EU MDR is extra than simply updating paperwork for regulatory compliance. It strengthens the gadget growth processes by requiring a design historical past file for all merchandise. It elevates medical knowledge necessities all through the life cycle of the gadget. And it requires producers to proactively monitor the protection and efficiency of the gadgets available in the market. As well as, compliance brings the provision chain into the scope of the standard administration system.
Producers of magnificence gadgets that even have medical gadgets of their product portfolio have the benefit of understanding the EU MDR necessities and will have already got a relationship established with a notified physique. Producers that at the moment don’t have a contract with a notified physique could expertise issue contracting with one as a result of restricted bandwidth of EU MDR designated notified our bodies and as a consequence of the truth that not all notified our bodies may have these magnificence gadgets in scope for certification.
Distributors that promote magnificence gadgets beneath their very own model title could need to rethink their enterprise mannequin, since they might develop into the authorized producer of the gadget beneath EU MDR and, because of this, they need to meet all the necessities for a medical gadget producer.
Many magnificence gadgets are offered on to shoppers. Design adjustments and options enhancements are usually frequent for these merchandise to cater to the shoppers’ curiosity and to remain forward of the competitors. Nevertheless, the regulation 2022/2346 doesn’t permit vital design adjustments to the product from June 2023 till the product is EU MDR compliant. This growth constraint could have an effect on the producer’s potential to introduce new product enhancements.
What Ought to Gadget Producers Do To Get Prepared For The EU MDR?
Producers of magnificence gadgets ought to begin planning for EU MDR compliance. As a primary step, start with an intensive hole evaluation of high quality administration system processes and product documentation, which can present a sign of the remediation effort wanted to help compliance. The evaluation will help a evaluation of the product portfolio; the target of this exercise might be to investigate the associated fee influence of compliance on income by product.
For the merchandise {that a} producer desires to take care of in the marketplace, an implementation plan should be developed contemplating compliance timelines, key milestones, and organizational influence on obtainable sources.
Distributors that promote magnificence gadgets — impacted by the EU MDR regulation — beneath their very own model title should consider whether or not they need to tackle the duties of a medical gadgets producer. Alternatively, distributors could select to vary their enterprise mannequin so as to meet the brand new EU MDR necessities.
About The Writer:
 Hilde Viroux is a medtech knowledgeable at PA Consulting and is an knowledgeable on the European Medical Units Regulation. She has a broad expertise in laws, high quality, manufacturing, provide chain, and challenge administration within the pharmaceutical and medical gadget business. She has an MSc in medical expertise regulatory affairs from Cranfield College within the U.Ok. and a BS in biochemistry engineering. Join together with her on LinkedIn.
Hilde Viroux is a medtech knowledgeable at PA Consulting and is an knowledgeable on the European Medical Units Regulation. She has a broad expertise in laws, high quality, manufacturing, provide chain, and challenge administration within the pharmaceutical and medical gadget business. She has an MSc in medical expertise regulatory affairs from Cranfield College within the U.Ok. and a BS in biochemistry engineering. Join together with her on LinkedIn.
Source link
